ALL PARTS OF THE MOTOR UNIT MATTER
Effective movement requires uninterrupted function of the entire motor unit—from the motor neuron cell body, along its axon, through the neuromuscular junction, to innervated muscle fibers.
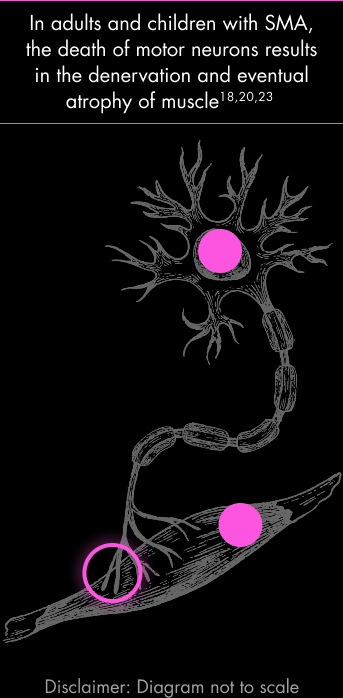
Disruptions along this pathway can lead to weakness and atrophy.18–21
MUSCLE: A CRITICAL CONTRIBUTOR TO SMA
In addition to the degradation of motor neurons, muscle wasting is central to the pathology of SMA.22,23 The overall burden of SMA for children and adults living with the disease is closely linked to functional deficits resulting from hypotonia and the loss of muscle.6
Research also suggests that a loss of SMN in skeletal muscle can have a direct impact, resulting in both cell- and non-cell-autonomous defects, such as central nucleation of myofibers, altered regeneration, and dysfunctional myogenesis.22
Lyza, 23
Living with SMA and advocating for progress
Modern intervention should consider the entire motor unit to adequately treat neuromuscular dysfunction.2,3
THE MUSCLE
MOSAIC
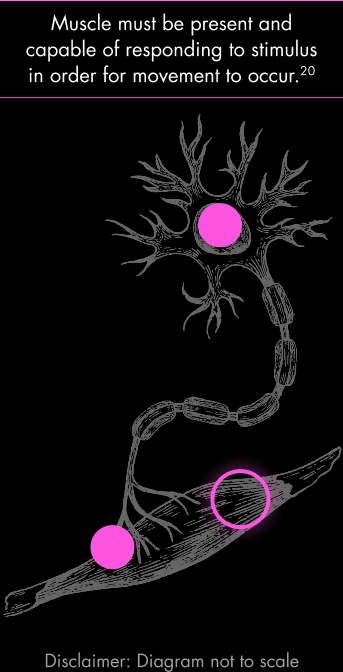
Despite progressive muscle wasting, people with SMA have some remaining intact muscle.3,20,24,25
Not all muscle fibers are equally affected by SMA. Some motor neurons maintain synaptic connections, preserving associated muscle fibers.3,20,24,25 Muscle biopsies in SMA consistently show a mosaic pattern: some fibers appear normal or even hypertrophic, while others are atrophic. This patchiness results from variable rates of denervation and ability of some motor units to compensate or persist longer than others.26,27
Individual motor neurons are affected differently, and atrophy is not uniform across muscle groups in individuals living with SMA.20,26
A unique opportunity exists to explore muscle-focused approaches to SMA treatment.
Putting the Muscle in Neuromuscular
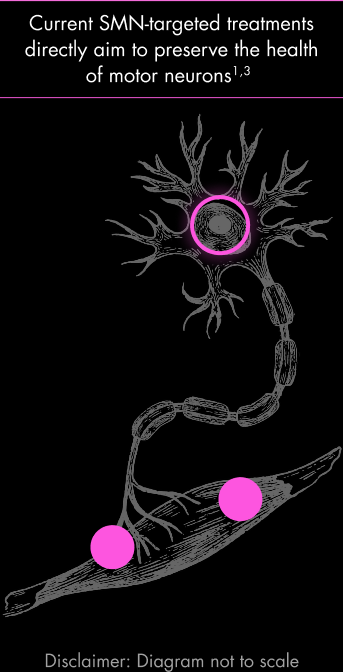
At the end of the motor neuron is a muscle that helps enable movement.20 SMN-targeted treatments were designed to address the motor neuron component of the disease and do not directly address the muscle.1,3
The Path Between Motor Neuron and Muscle
LEARN MORE ABOUT A POTENTIAL TARGET IN SMA TO ADDRESS MUSCLE DYSFUNCTION
References
- Antonaci L, Pera MC, Mercuri E. New therapies for spinal muscular atrophy: where we stand and what is next. Eur J Pediatr. 2023;182(7):2935-2942.
- Abati E, Manini A, Comi GP, Corti S. Inhibition of myostatin and related signaling pathways for the treatment of muscle atrophy in motor neuron diseases. Cell Mol Life Sci. 2022;79(7):374.
- Day JW, Howell K, Place A, et al. Advances and limitations for the treatment of spinal muscular atrophy. BMC Pediatr. 2022;22(1):632.
- Gnazzo M, Pisanò G, Baldini V, et al. Myostatin modulation in spinal muscular atrophy: A systematic review of preclinical and clinical evidence. Int J Mol Sci. 2025;26(12).
- Schroth M, Deans J, Arya K, et al. Spinal muscular atrophy update in best practices: recommendations for diagnosis considerations. Neurol Clin Pract. 2024;14(4):e200310.
- Parsons JA, Land N, Maravic MC, et al. Remaining burden of spinal muscular atrophy among treated patients: A survey of patients and caregivers. Ann Clin Transl Neurol. 2025;12(10):2020-2035.
- Finkel RS, Farrar MA, Saito K, et al. Final Safety and Efficacy Data From the SHINE Study in Participants With Infantile-Onset and Later-Onset SMA. Poster presented at: Annual Cure SMA Research and Clinical Care Meeting; June 2024; Austin, TX.
- Servais L, Oskoui M, Day J, et al. SUNFISH Parts 1 and 2: 4-year Efficacy and Safety Data of Risdiplam in Types 2 and 3 Spinal Muscular Atrophy (SMA). Poster presented at: Muscular Dystrophy Association (MDA) Clinical and Scientific Conference; March 2025; Dallas, TX.
- Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N Engl J Med. 2018;378(7):625-635.
- A Study for Participants With Spinal Muscular Atrophy (SMA) Who Previously Participated in Nusinersen (ISIS 396443) Investigational Studies (SHINE). ClinicalTrials.gov identifier: NCT02594124. Updated September 27, 2024. Accessed December 10, 2025. https://clinicaltrials.gov/study/NCT02594124
- Ch’ng GS, Koh K, Ahmad-Annuar A, et al. A mixed method study on the impact of living with spinal muscular atrophy in Malaysia from patients’ and caregivers’ perspectives. Orphanet J Rare Dis. 2022;17(1):200.
- Wan HWY, Carey KA, D’Silva A, Kasparian NA, Farrar MA. “Getting ready for the adult world”: how adults with spinal muscular atrophy perceive and experience healthcare, transition and well-being. Orphanet J Rare Dis. 2019;14(1):74.
- Duong T, Braid J, Staunton H, et al. Understanding the relationship between the 32-item motor function measure and daily activities from an individual with spinal muscular atrophy and their caregivers’ perspective: a two-part study. BMC Neurol. 2021;21(1):143.
- Rodriguez-Torres RS, Uher D, Gay EL, et al. Measuring fatigue and fatigability in spinal muscular atrophy (SMA): challenges and opportunities. J Clin Med. 2023;12(10).
- Binz C, Schreiber-Katz O, Kumpe M, et al. An observational cohort study on impact, dimensions and outcome of perceived fatigue in adult 5q-spinal muscular atrophy patients receiving nusinersen treatment. J Neurol. 2021;268(3):950-962.
- Welsh EF. Belter L, Whitmire SM, Curry M, Schroth M. Unmet Needs Among Adults Living with Spinal Muscular Atrophy in the United States. Poster presented at: Muscular Dystrophy Association (MDA) Clinical and Scientific Conference; March 2025; Dallas, TX.
- McGraw S, Qian Y, Henne J, Jarecki J, Hobby K, Yeh W-S. A qualitative study of perceptions of meaningful change in spinal muscular atrophy. BMC Neurol. 2017;17(1):68.
- Mercuri E, Finkel RS, Muntoni F, et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28(2):103-115.
- Boyd PJ, Gillingwater TH. Axonal and Neuromuscular Junction Pathology in Spinal Muscular Artophy. In: Sumner CJ, Paushkin S, Ko CP, eds. Spinal Muscular Atrophy: Disease Mechanisms and Therapy. Elsevier; 2017:133-151.
- Le Verche V, Sunshine SS, Hammers D. Skeletal Muscle in Spinal Muscular Atrophy As an Opportunity for Therapeutic Intervention. In: Sumner CJ, Paushkin S, Ko CP, eds. Spinal Muscular Atrophy: Disease Mechanisms and Therapy. Elsevier; 2017:341-356.
- Deguise MO, Patitucci TN, Ebert AD, Lorson CL, Kothary R. Contributions of Different Cell Types to Spinal Muscular Atrophy Pathogenesis. In: Sumner CJ, Paushkin S, Ko CP, eds. Spinal Muscular Atrophy: Disease Mechanisms and Therapy. Elsevier; 2017:167-181.
- Jha NN, Kim J-K, Her Y-R, Monani UR. Muscle: an independent contributor to the neuromuscular spinal muscular atrophy disease phenotype. JCI Insight. 2023;8(18).
- Wirth B, Mendoza-Ferreira N, Torres-Benito L. Spinal Muscular Artophy Disease Modifiers. In: Sumner CJ, Paushkin S, Ko CP, eds. Spinal Muscular Atrophy: Disease Mechanisms and Therapy. Elsevier; 2017:191-210.
- Durmus H, Yilmaz R, Gulsen-Parman Y, et al. Muscle magnetic resonance imaging in spinal muscular atrophy type 3: Selective and progressive involvement. Muscle Nerve. 2017;55(5):651-656.
- Deymeer F. Natural history of SMA IIIb. Neurology. August 26, 2008.
- Buchthal F, Olsen PZ. Electromyography and muscle biopsy in infantile spinal muscular atrophy. Brain. 1970;93(1):15-30.
- Perez-Garcia MJ, Kong L, Sumner CJ, Tizzano EF. Developmental Aspects and Pathological Findings in Spinal Muscular Atrophy. In: Sumner CJ, Paushkin S, Ko CP, eds. Spinal Muscular Atrophy: Disease Mechanisms and Therapy. Elsevier; 2017:21-42.
- McCarthy JJ, Esser KA. Anabolic and catabolic pathways regulating skeletal muscle mass. Curr Opin Clin Nutr Metab Care. 2010;13(3):230-235.
- Lee S-J. Targeting the myostatin signaling pathway to treat muscle loss and metabolic dysfunction. J Clin Invest. 2021;131(9).
- Wagner KR. The elusive promise of myostatin inhibition for muscular dystrophy. Curr Opin Neurol. 2020;33(5):621-628.
- Lee S-J, Bhasin S, Klickstein L, Krishnan V, Rooks D. Challenges and future prospects of targeting myostatin/activin A signaling to treat diseases of muscle loss and metabolic dysfunction. J Gerontol A Biol Sci Med Sci. 2023;78(Suppl 1):32-37.